MeiraGTx announces Q3 earnings per share of 62 cents, compared to a loss of 55 cents in the previous year.
Q3 Financial Performance: The company reported Q3 revenue of $410,000, a significant decrease from $10.9 million last year, while announcing a strategic collaboration with Eli Lilly focused on gene therapy for LCA4, a severe inherited retinopathy.
Gene Therapy Advancements: The AAV-AIPL1 program has shown promising results, with all 11 treated children gaining vision, highlighting the potential of gene therapy for severe genetic conditions when administered early.
Clinical Program Progress: The company is on track with its Phase 2 study of AAV-hAQP1 for radiation-induced xerostomia, expecting to complete enrollment by year-end and potentially file for a BLA in early 2027.
New Initiatives and Collaborations: The company is preparing to initiate a Phase 3 study for AAV-GAD in Parkinson's disease and has optimized its riboswitch program for leptin deficiency, while also developing a treatment for BBS10-associated retinal dystrophy in collaboration with a philanthropic organization.
Trade with 70% Backtested Accuracy
Analyst Views on MGTX
About MGTX
About the author

- Biotech Deal Trends: Pharmaceutical companies are increasingly prioritizing late-stage assets with clinical validation over early-stage projects, positioning firms like Oncotelic Therapeutics as strategic assets capable of accelerating commercialization pathways.
- IP Expansion: Oncotelic Therapeutics announced enhancements to its OT-101 platform's international intellectual property protection in neurology and oncology, which will bolster its competitive edge in treating complex biological mechanisms.
- Strengthened Clinical Foundation: OT-101 has demonstrated strong performance in multiple oncology trials, recently securing a patent for Parkinson's disease in Australia, laying a solid foundation for future commercialization efforts.
- Cross-Indication Opportunities: Oncotelic's R&D strategy focuses on the TGF-β signaling pathway, which is significant in both oncology and neurological diseases, indicating potential value and strategic flexibility across multiple markets.
- Strategic Transformation: Pharmaceutical companies are increasingly prioritizing late-stage assets with clinical validation over early-stage projects, positioning Oncotelic Therapeutics as a focal point due to its multiple clinical-stage programs in oncology and CNS, aligning with current M&A priorities.
- IP Expansion: Oncotelic Therapeutics announced enhancements to its global intellectual property protection for the OT-101 platform, particularly in drug delivery for neurology and oncology, which will bolster its competitive edge and strategic value in the market.
- Market Demand: The rising need for treatments for CNS disorders such as Alzheimer's and Parkinson's highlights the potential of Oncotelic's OT-101 platform, which targets the TGF-β signaling pathway to address these significant unmet medical needs.
- Capital Efficiency: The biotech industry is increasingly emphasizing capital efficiency, and Oncotelic's strategy of repositioning clinically validated mechanisms into additional therapeutic indications reduces risk while maximizing the value of prior investments, enhancing its market appeal.
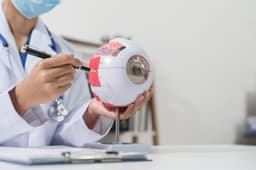
MeiraGTx and Eli Lilly Collaboration: MeiraGTx Holdings has entered a strategic partnership with Eli Lilly, granting exclusive rights to its AAV-AIPL1 program for treating Leber congenital amaurosis 4, with an upfront payment of $75 million and potential milestone payments exceeding $400 million.
Clinical Success of AAV-AIPL1: Clinical trials showed that all 11 children treated with AAV-AIPL1 gained vision, with additional improvements in communication, behavior, learning, and social integration.
Riboswitch Technology: The collaboration includes access to MeiraGTx’s riboswitch technology for gene editing in ophthalmology, allowing precise control over therapeutic protein production through oral dosing.
SanegeneBio and Eli Lilly Partnership: SanegeneBio has also partnered with Eli Lilly to advance RNAi candidates for metabolic diseases, with potential milestone payments up to $1.2 billion and a focus on developing therapies that can be administered infrequently.

Collaboration Announcement: MeiraGTx (MGTX) shares rose approximately 18% in premarket trading after announcing a strategic collaboration with Eli Lilly (LLY) to develop gene therapies for eye diseases, specifically targeting Leber congenital amaurosis 4 (LCA4).
Exclusive Rights and Financial Terms: Under the agreement, Eli Lilly will receive exclusive global rights to MGTX's AAV-AIPL1 gene therapy program and other gene therapy technologies, with MeiraGTx set to receive $75 million upfront and over $400 million based on milestone achievements, along with tiered royalties on future sales.

Real-time Intelligence: Benzinga Pro offers the fastest news alerts for traders, helping them stay informed and make timely decisions in the stock market.
Exclusive Content: The platform provides exclusive stories and insights generated by Benzinga reporters, enhancing the trading experience for its users.
Community Engagement: Over 10,000 serious traders are part of the Benzinga Pro community, indicating a robust network for sharing market intelligence.
Market Winning Tools: Benzinga Pro is designed to equip traders with the tools and information necessary to succeed in the markets daily.
Insider Trading Activity: Richard Giroux, CFO & COO of $MGTX, sold 24,000 shares for approximately $204,480, marking a 2.7% reduction in his holdings, with no insider purchases reported in the last six months.
Analyst Ratings and Institutional Activity: Recent reports show that 59 institutional investors increased their stakes in $MGTX, while analysts have issued two buy ratings and set a median price target of $24.0 for the stock.







