Biohaven Ltd's Depression Drug Trial Fails to Meet Primary Endpoint, Shares Plunge 14.3%
Written by Emily J. Thompson, Senior Investment Analyst
Updated: Dec 26 2025
0mins
Should l Buy BHVN?
Source: Benzinga
- Drug Trial Failure: Biohaven Ltd announced that its depression drug BHV-7000 failed to significantly reduce depressive symptoms in a six-week clinical trial, causing shares to plummet 14.3% to $9.27 in pre-market trading, reflecting a substantial decline in market confidence regarding its R&D capabilities.
- Severe Market Reaction: The news of the trial's failure triggered panic among investors, leading to a rapid decline in Biohaven's stock price during pre-market trading, indicating the high sensitivity of the biopharmaceutical sector to clinical outcomes, which may impact the company's future financing and R&D plans.
- Increased Competitive Pressure: With Biohaven's drug trial failure, market attention shifts to other competitors, particularly in the depression treatment space, potentially leading investors to reassess the market outlook and investment value of related companies.
- Wider Industry Implications: Biohaven's failure could have a ripple effect across the biopharmaceutical industry, particularly in terms of R&D investments and clinical trial designs, prompting other companies to more cautiously evaluate their drug development strategies.
Trade with 70% Backtested Accuracy
Stop guessing "Should I Buy BHVN?" and start using high-conviction signals backed by rigorous historical data.
Sign up today to access powerful investing tools and make smarter, data-driven decisions.
Analyst Views on BHVN
Wall Street analysts forecast BHVN stock price to rise
13 Analyst Rating
9 Buy
4 Hold
0 Sell
Moderate Buy
Current: 11.150
Low
11.00
Averages
18.00
High
30.00
Current: 11.150
Low
11.00
Averages
18.00
High
30.00
About BHVN
Biohaven Ltd. is a biopharmaceutical company focused on the discovery, development and commercialization of treatments in key therapeutic areas, including immunology, neuroscience, and oncology. It is advancing its portfolio of therapeutics, leveraging its drug development experience and multiple proprietary drug development platforms. Its clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; TRPM3 antagonism for migraine and neuropathic pain; TYK2/JAK1 inhibition for neuroinflammatory disorders; glutamate modulation for obsessive-compulsive disorder (OCD) and spinocerebellar ataxia (SCA); myostatin inhibition for neuromuscular and metabolic diseases, including spinal muscular atrophy (SMA) and obesity; antibody recruiting bispecific molecules; and antibody drug conjugates for cancer. Its advanced product candidate from its glutamate receptor antagonist platform is troriluzole.
About the author

Emily J. Thompson
Emily J. Thompson, a Chartered Financial Analyst (CFA) with 12 years in investment research, graduated with honors from the Wharton School. Specializing in industrial and technology stocks, she provides in-depth analysis for Intellectia’s earnings and market brief reports.
- Watts Water Upgrade: Keybanc analyst Jeffrey Hammond upgraded Watts Water Technologies Inc (NYSE:WTS) from Sector Weight to Overweight with a price target of $340, reflecting confidence in its growth potential, closing at $289.31 on Tuesday.
- Biohaven Price Target Raised: RBC Capital analyst Leonid Timashev upgraded Biohaven Ltd (NYSE:BHVN) from Sector Perform to Outperform, raising the price target from $9 to $22, indicating optimism about its product prospects, with shares closing at $12.68 on Tuesday.
- Ulta Beauty Rating Upgrade: Raymond James analyst Olivia Tong upgraded Ulta Beauty Inc (NASDAQ:ULTA) from Outperform to Strong Buy, increasing the price target from $605 to $790, showcasing strong confidence in its market performance, with shares closing at $675.62 on Tuesday.
- Albemarle Price Target Increase: Truist Securities analyst Peter Osterland upgraded Albemarle Corp (NYSE:ALB) from Hold to Buy, boosting the price target from $125 to $205, signaling positive expectations for its future performance, with shares closing at $172.54 on Tuesday.
See More

- Rating Upgrade: RBC Capital Markets upgraded Biohaven from sector perform to outperform, with analyst Timashev noting that new data has reduced uncertainty around the company's epilepsy drug, setting a price target of $22, implying a 74% upside.
- Data Support: Recent January data indicates that Biohaven's Kv7 drug shows activity in the central nervous system, alleviating investors' worst-case concerns ahead of upcoming late-stage trials, thus boosting market confidence.
- Market Potential: Timashev believes the Kv7 modulator may demonstrate a better safety profile than competitors and has the potential to be a fast follower in a market that can support multiple branded drugs, enhancing Biohaven's competitive edge.
- Financial Outlook: While Biohaven still carries a relatively high risk profile, the analyst highlights that the improved financial position and discount relative to the platform's opportunity create an attractive setup for catalysts in 2H26, with successful clinical trials potentially pushing the stock price to $30, indicating nearly 137% upside.
See More
- Stock Surge: Biohaven's shares rose 3.42% to $12.10 in after-hours trading on Wednesday, reflecting positive market sentiment towards its clinical data despite a 67.11% decline over the past year.
- SEC Filing Disclosure: The company filed an investor presentation with the SEC on Monday, showcasing portfolio progress at the 44th Annual J.P. Morgan Healthcare Conference, indicating compliance with transparency and fair disclosure regulations.
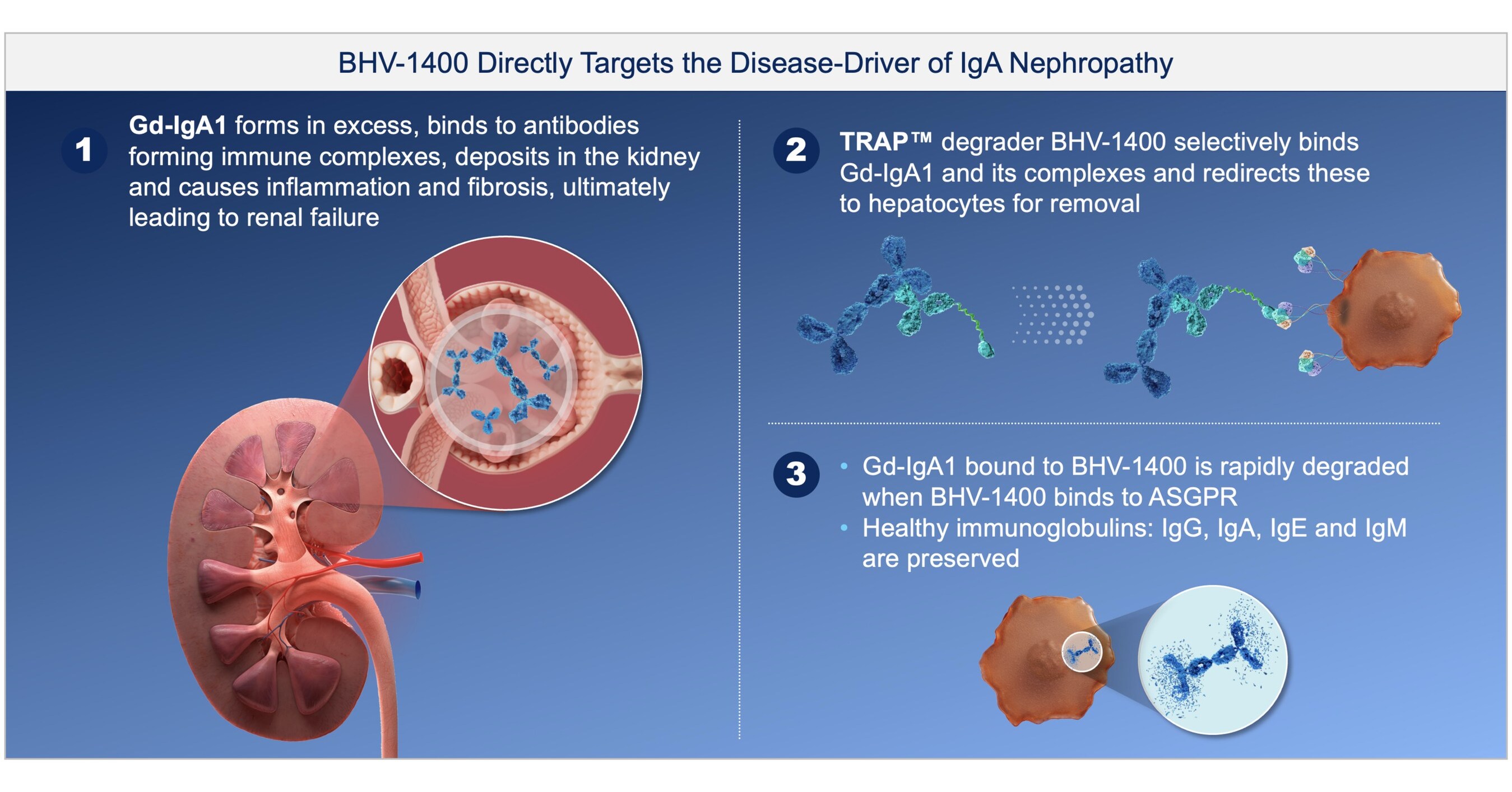
- Clinical Data Highlights: BHV-1400 achieved over 60% mean Gd-IgA1 reductions in Phase 1 studies for IgA nephropathy, with two patients showing clinical improvements, underscoring the drug's potential in treating immunological diseases.
- Future Catalysts: Biohaven anticipates pivotal trial results in 2026 for multiple drugs, including those targeting obesity and Graves' disease, demonstrating the company's ongoing commitment to R&D and future growth potential.
See More
- Portfolio Progress: At the 44th Annual J.P. Morgan Healthcare Conference, Biohaven showcased its broad portfolio progress, emphasizing its efforts in developing innovative therapies for both rare and common diseases, reflecting its ongoing innovation in the biopharmaceutical sector.
- Clinical Stage Focus: As a global clinical-stage biopharmaceutical company, Biohaven's focus on discovering and developing life-changing therapies indicates its strategic positioning and market potential, which may attract increased investor interest.
- Enhanced Transparency: By providing a slide presentation at the conference, Biohaven has improved information transparency with investors, which could help boost market confidence and shareholder value.
- Industry Influence: By participating in this significant healthcare conference, Biohaven not only showcased its R&D achievements but also strengthened connections with industry leaders and potential partners, potentially paving the way for future collaborations and market expansion.
See More
- IgA Nephropathy Progress: Biohaven's BHV-1400 TRAP degrader demonstrated over 60% selective reduction of disease-causing Gd-IgA1 in IgA nephropathy patients within weeks, leading to complete resolution of hematuria and improved kidney function, highlighting its therapeutic potential.
- Graves' Disease Treatment: The first clinical use of BHV-1300 in Graves' disease patients resulted in complete suppression of pathogenic antibodies and normalization of thyroid hormones within weeks, with maximum IgG reductions of 87%, setting the stage for pivotal studies.
- Epilepsy Treatment Breakthrough: Biohaven's selective Kv7 channel activator Opakalim showed significant reductions in seizure frequency in an open-label extension study, with over 50% of participants achieving a ≥50% reduction after 6 months, indicating its transformative potential in epilepsy management.
- Obesity Study Initiation: Biohaven launched a Phase 2 study in Q4 2025 targeting obesity with myostatin-activin inhibitor Taldefgrobep, aiming for high-quality weight loss, with topline results expected in 2026.
See More

- Conference Appearance: Biohaven CEO Vlad Coric will present at the J.P. Morgan Healthcare Conference on January 12, 2026, in San Francisco, showcasing the company's latest advancements in biopharmaceuticals, which is expected to attract investor and industry attention.
- Innovative Portfolio: Biohaven focuses on key therapeutic areas such as immunology, obesity, neuroscience, and oncology, advancing multiple clinical and preclinical programs, including Kv7 ion channel modulators, aimed at providing life-changing treatments for patients.
- Market Potential: The company is leveraging its proprietary drug development platforms to commercialize innovative therapies, which is expected to lay the groundwork for future revenue growth, particularly in the rapidly evolving biopharmaceutical market.
- Risk Advisory: Biohaven cautions investors that future development and marketing approvals involve substantial risks and uncertainties, with actual results potentially differing significantly from expectations, necessitating close attention to FDA regulatory developments.
See More





