Opus Genetics Advances as Eye Disease Research Continues Unchanged
Clinical Trial Update: Opus Genetics' shares rose after the company announced it will continue its Phase 1/2 BEST1 clinical trial for the eye disease therapy OPGx-BEST1 without modifications, following a safety review by an Independent Data Monitoring Committee.
Safety Review Findings: The Independent Data Monitoring Committee recommended the continuation of the trial, which targets patients with Best disease, based on a four-week safety data analysis from the first participant.
Company Background: Opus Genetics was formed after Ocuphire Pharma acquired it in an all-stock deal last year, focusing on gene therapy for eye diseases.
CEO's Confidence: CEO George Magrath expressed confidence in the program's advancement following the positive safety review and recommendation from the IDMC.
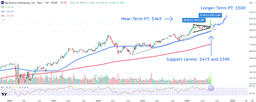
Trade with 70% Backtested Accuracy
Analyst Views on IRD
About IRD
About the author

- Funding Plan: Opus Genetics announced plans to raise $25 million through a private placement led by Boston's Adage Capital Management, expected to close on February 18, 2026, indicating proactive capital market engagement.
- Stock Issuance Details: The company intends to sell over 7.3 million shares of Series B Non-Voting Convertible Preferred Stock at $3.39 per share, with each share convertible into common stock, enhancing potential returns for investors.
- Use of Proceeds: The net proceeds from this offering will be directed towards advancing gene therapy programs and other operational activities, reflecting the company's commitment to future R&D and responsiveness to market demands.
- Cash Flow Status: Opus Genetics disclosed a year-end cash balance of $70 million, which is expected to sustain operations into H1 2028, demonstrating financial robustness and sustainable growth potential.
- Private Placement Agreement: Opus Genetics has entered into a securities purchase agreement to issue 7,374,632 shares of Series B Non-Voting Convertible Preferred Stock at $3.39 per share, with expected gross proceeds of $25 million, enhancing the company's financial strength to support its gene therapy initiatives.
- Clear Use of Funds: The proceeds from this financing will be utilized to advance gene therapy clinical programs and for general working capital, with a projected cash balance of $70 million by year-end 2025, sufficient to fund operations into the first half of 2028, demonstrating the company's confidence in future growth.
- Shareholder Approval Requirement: The private placement is contingent upon shareholder approval for an increase in authorized common stock to facilitate the conversion of Series B Preferred Stock, reflecting strategic considerations in capital structure adjustments aimed at enhancing shareholder value.
- Legal Compliance Measures: Opus Genetics has entered into a registration rights agreement with investors, committing to file a registration statement with the SEC to ensure compliance with securities laws for the shares issued in the private placement, indicating the company's focus on regulatory adherence and enhancing investor confidence.

- Insider Purchase: Cam Gallagher of Opus Genetics acquired 164,000 shares of IRD at $1.97 each on Wednesday, totaling an investment of $323,693, reflecting confidence in the company's future prospects.
- Investment Return: Gallagher's investment is currently up approximately 18.1%, based on today's trading high of $2.33, indicating a positive market response and potential growth outlook for IRD.
- Market Performance: Opus Genetics shares rose about 8.7% on Friday, reflecting optimistic sentiment among investors regarding the company's outlook, potentially driven by Gallagher's purchase.
- CEO Acquisition: Additionally, CEO Dwayne L. Hyzak purchased 3,712 shares of MSC Income Fund at $13.43 each for a total of $49,852, demonstrating ongoing confidence in the fund's performance.
- Equity Incentive Plan: Opus Genetics approved an equity award of 50,000 stock options to a new employee under its 2021 Inducement Plan, aimed at attracting top talent and strengthening its team to drive innovation in gene therapy.
- Option Details: The options have an exercise price equal to the fair market value of the company's common stock on the grant date, vesting over four years with 25% vesting after one year and the remaining 75% in quarterly installments, ensuring long-term employee retention aligned with company goals.
- Compliance Assurance: This equity award complies with Nasdaq Listing Rule 5635(c)(4), ensuring that the company adheres to transparent compliance procedures while attracting talent, thereby enhancing investor confidence.
- Company Background: Opus Genetics focuses on developing gene therapies to restore vision for patients with inherited retinal diseases, with a pipeline of seven AAV-based programs, showcasing the company's strong R&D capabilities and market potential in the biopharmaceutical sector.

- Conference Presentation: Opus Genetics CEO George Magrath will present at the J.P. Morgan Healthcare Conference on January 15, 2026, showcasing the company's latest advancements in gene therapy, which is expected to attract investor interest.
- Pipeline Development: The company is developing seven AAV-based programs, including treatments for LCA5 and BEST1-related retinal degeneration, aimed at addressing the underlying genetic causes of severe retinal disorders, potentially offering new therapeutic options for patients.
- Therapeutic Progress: Opus Genetics is also advancing Phentolamine Ophthalmic Solution, targeting pharmacologically induced mydriasis, with additional indications for presbyopia and low-light visual disturbances in late-stage development, indicating the company's diversified approach in the ophthalmology sector.
- Company Overview: Based in Research Triangle Park, NC, Opus Genetics focuses on developing gene therapies to restore vision for patients with inherited retinal diseases, demonstrating its innovative potential in the biopharmaceutical industry.

- Leadership Presentation: Opus Genetics CEO George Magrath will speak at the J.P. Morgan Healthcare Conference on January 15, 2026, showcasing the company's latest advancements in gene therapy, which is expected to attract investor interest and enhance the company's visibility.
- Technical Focus: Opus Genetics specializes in developing gene therapies for inherited retinal diseases, with a pipeline that includes seven AAV-based programs aimed at providing one-time treatments to address the underlying genetic causes of severe retinal disorders, potentially transforming patient quality of life.
- Product Development: The company is advancing Phentolamine Ophthalmic Solution, which is not only approved for pharmacologically induced mydriasis but also in late-stage development for presbyopia and low-light visual disturbances, indicating its diversified approach in the ophthalmic sector.
- Market Positioning: By focusing on addressing the root causes of severe retinal diseases, Opus Genetics is well-positioned to capture a share in the rapidly growing biopharmaceutical market as demand for gene therapies increases.