IMUNON R&D Day: Chance to Listen to Clinical Trial Researchers Discuss the Promising Impact of IMNN-001 on Ovarian Cancer Therapy
Event Announcement: IMUNON, Inc. will host an R&D Day on November 10, 2025, at 8:00 a.m. ET in New York City, focusing on their DNA-mediated immunotherapy for advanced ovarian cancer, featuring discussions with leading clinical trial investigators.
Clinical Trial Insights: Presentations will cover the urgent need for new ovarian cancer treatments, data from the OVATION 2 trial, results from the Phase 2 minimal residual disease study, and the statistical design of the ongoing Phase 3 OVATION 3 trial.
Company Overview: IMUNON is a clinical-stage biotechnology company developing innovative treatments using non-viral DNA technology, with its lead program, IMNN-001, aimed at localized treatment of advanced ovarian cancer.
Forward-Looking Statements: The company cautions that forward-looking statements regarding clinical trials and product potential involve risks and uncertainties, and they do not assume any obligation to update these statements.
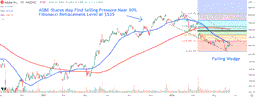
Trade with 70% Backtested Accuracy
Analyst Views on IMNN
About IMNN
About the author

- Financing Agreement Reached: IMUNON has entered into a securities purchase agreement with a healthcare-focused institutional investor to sell 1,939,114 shares of common stock and associated warrants, with expected gross proceeds of approximately $7 million, enhancing the company's liquidity to support its clinical-stage immunotherapy development.
- Warrant Details: Each share is priced at $3.61, with warrants having an exercise price of $3.482, which will be exercisable immediately and expire in five years, providing the company with additional capital sources to drive future R&D efforts.
- Market Reaction Anticipated: The offering is expected to close on December 31, 2025, and if successful, will help IMUNON maintain compliance with Nasdaq requirements and avoid potential delisting risks, thereby boosting investor confidence.
- Registration Statement Background: The securities are being offered under a Form S-3 registration statement declared effective on May 22, 2024, ensuring compliance and laying the groundwork for future financing activities, demonstrating the company's flexibility and adaptability in capital markets.
- Significant Clinical Progress: IMUNON initiated its pivotal Phase 3 OVATION3 study in 2025, evaluating IMNN-001 in combination with standard chemotherapy, demonstrating strong clinical momentum that could redefine treatment standards for ovarian cancer.
- Survival Extension: The Phase 2 OVATION2 study revealed a 13-month median overall survival extension in the intent-to-treat population with IMNN-001, and in patients receiving PARP inhibitors, the median survival has not yet been reached, indicating its potential therapeutic advantage.
- Immunotherapy Promise: New data indicates that IMNN-001 activates the tumor microenvironment, leading to IL-12 production and enhanced T cell functions, showcasing its broad applicability in frontline ovarian cancer treatment.
- Financial Discipline: IMUNON maintained financial discipline in 2025, planning to invest $30 million in the OVATION3 trial, reflecting the company's confidence in its R&D and market opportunities.
- Significant Clinical Progress: IMUNON initiated its pivotal Phase 3 OVATION 3 study in 2025, evaluating IMNN-001 in combination with standard chemotherapy, demonstrating strong clinical outcomes that could redefine treatment standards for ovarian cancer.
- Survival Extension: In the Phase 2 OVATION 2 study, IMNN-001 showed a 13-month median overall survival extension in the intent-to-treat population, with survival not yet reached in patients receiving PARP inhibitors, indicating its potential in ovarian cancer treatment.
- Promise of Immunotherapy: New data indicates that IMNN-001 activates macrophages in the tumor microenvironment, leading to IL-12 production and enhanced T cell cytotoxic functions, showcasing its promise as a frontline therapy.
- Financial Discipline: IMUNON maintained financial discipline in 2025, planning to invest $30 million in the OVATION 3 trial, reflecting the company's confidence in future growth and commitment to shareholder value.
R&D Day Highlights: IMUNON showcased significant progress in its Phase 3 OVATION 3 Study for IMNN-001, a potential frontline immunotherapy for advanced ovarian cancer, emphasizing strong survival data and a favorable safety profile.
Financial Performance: The company reported a net loss of $3.4 million for Q3 2025, a decrease from the previous year, with operating expenses also down by 30%, indicating improved financial management.
Clinical Advancements: IMNN-001 demonstrated the ability to create a "hot" tumor microenvironment, enhancing immune responses and showing promising results in ongoing clinical trials, including a notable median overall survival benefit.
PlaCCine Technology Development: IMUNON is advancing its PlaCCine DNA vaccine technology, presenting positive proof-of-concept data at various conferences, and is seeking partnerships to enhance its vaccine development efforts.

Webcast Event: IMUNON, Inc. hosted a public webcast featuring cancer experts discussing the potential of their DNA-mediated immunotherapy, IMNN-001, for treating advanced ovarian cancer, with recordings and slides available on their website.
Company Overview: IMUNON is a clinical-stage biotechnology company focused on innovative treatments using non-viral DNA technology, including their lead program IMNN-001 and a COVID-19 booster vaccine (IMNN-101).
Clinical Trials: IMNN-001 has completed a Phase 2 trial and is currently in Phase 3 trials, demonstrating the company's commitment to advancing therapies for difficult-to-treat conditions.
Forward-Looking Statements: The company issued a caution regarding forward-looking statements, highlighting the risks and uncertainties associated with clinical trials and the development of their therapies.