Soligenix Publishes Phase 2a Study Results for SGX945 in Behcet's Disease
Soligenix announced that the results from its Phase 2a proof of concept study evaluating SGX945, or dusquetide, in the treatment of Behcet's Disease have been published in Rheumatology (Oxford). The Phase 2a study, evaluating control of oral ulcers in Behcet's Disease, reported beneficial effects for 7 of 8 patients, over the 4 weeks of treatment as well as a potentially enduring effect through the 4 weeks of follow-up. Using the Phase 3 study of apremilast as a baseline for comparison, this open-label study indicated that the area under the curve, average number of oral ulcers, and improvements in oral pain for SGX945 were similar to outcomes obtained in the apremilast study. Notably, outcomes in weeks 5 through 8 continued to show similar outcomes, even though apremilast treatment was continued through this period whereas SGX945 treatment was stopped at Week 4, per study design. SGX945 was well-tolerated with no treatment-related adverse events. Common adverse events for apremilast included diarrhea - 41% of patients -, nausea - 19% of patients - and headache - 14% of patients -, none of which were observed with SGX945.
Trade with 70% Backtested Accuracy
Analyst Views on SNGX
About SNGX
About the author

- Presentation Schedule: Soligenix's CEO, Dr. Christopher J. Schaber, will present at the BIO Investment & Growth Summit on March 2, 2026, at 3 PM in Miami, showcasing the company's advancements in rare disease treatments, which is expected to attract investor interest.
- Product Development Progress: The company is advancing its HyBryte™ (SGX301) into Phase 3 clinical trials, having successfully completed the second Phase 2 study, with plans to seek global regulatory approvals that could significantly enhance market opportunities and revenue potential.
- Vaccine Development Support: Soligenix's Public Health Solutions segment includes the development of vaccines like RiVax®, supported by government grants from NIAID and BARDA, highlighting the company's strategic positioning and collaboration potential in the biodefense sector.
- Future Outlook and Risks: Despite promising clinical trial results for HyBryte™, the company faces regulatory challenges from the FDA and EMA, indicating that future product development and commercialization efforts remain uncertain, necessitating close monitoring of market dynamics and policy changes.
- Presentation Schedule: Soligenix's CEO, Dr. Christopher J. Schaber, will present at the BIO Investment & Growth Summit on March 2, 2026, at 3 PM in Miami, showcasing the company's advancements in treating rare diseases, which is expected to attract investor interest.
- Product Development Progress: The company is advancing HyBryte™ (SGX301) as a novel photodynamic therapy, having successfully completed the second Phase 3 clinical trial, and if regulatory approvals are obtained, it will provide new treatment options globally, significantly enhancing the company's competitiveness in the biopharmaceutical sector.
- Public Health Solutions: Soligenix's Public Health Solutions segment is developing vaccine candidates like RiVax®, supported by government grants from NIAID and BARDA, demonstrating the company's strategic positioning in addressing bioterror threats and strengthening its market presence.
- Future Outlook and Risks: Despite achieving statistically significant results in HyBryte™ clinical trials, the company faces regulatory challenges from the FDA and EMA, and future product development and commercialization efforts may be impacted by clinical trial delays and funding shortages, necessitating careful navigation of market competition.
- Market Potential Assessment: Soligenix anticipates that annual net sales of HyBryte™ in the U.S. will exceed $90 million, with the total addressable worldwide CTCL market estimated at over $250 million, indicating strong market demand and potential returns.
- Robust Financing Strategy: As of September 30, 2025, Soligenix reported approximately $10.5 million in cash, along with $500,000 in non-dilutive funding, ensuring the company can meet its development goals through 2026, thereby boosting investor confidence.
- Clinical Trial Progress: The company is advancing its confirmatory Phase 3 clinical trial for HyBryte™, with multiple significant milestones expected in 2026, which will pave the way for future market authorizations and enhance its competitive position.
- Strategic Partnership Opportunities: Soligenix is evaluating partnerships in international markets, aiming to accelerate the global marketing authorization process for HyBryte™ through collaborations with companies that have similar reputations and expertise, further expanding its business footprint.
- Clinical Trial Progress: As of February 10, 2026, Soligenix has enrolled 66 patients in the Phase 3 FLASH2 clinical trial for HyBryte™, with top-line results expected in the second half of 2026, enhancing confidence in the interim analysis and potentially boosting the company's competitiveness in the cutaneous T-cell lymphoma market.
- Treatment Success Rate: In an open-label study, 75% of early-stage CTCL patients achieved 'Treatment Success' after 18 weeks of HyBryte™ treatment, indicating the therapy's potential in chronic cancer treatment and laying a foundation for future market promotion.
- Market Opportunity Assessment: Peak annual sales of HyBryte™ in the U.S. are expected to exceed $90 million, with the total addressable worldwide CTCL market estimated at over $250 million, highlighting the company's commercial potential in the rare disease sector.
- Financing Strategy: With approximately $10.5 million in cash reported as of September 30, 2025, Soligenix is well-positioned to meet its funding needs for advancing development programs in 2026, demonstrating ongoing investment and growth potential in rare disease therapeutics.
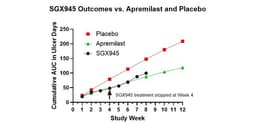
- Clinical Trial Results: Soligenix's SGX945 demonstrated significant improvement in 7 out of 8 patients in a Phase 2a trial for Behçet's Disease, indicating potential enduring effects after 4 weeks of treatment, which could enhance patient quality of life.
- Efficacy Comparison: After 4 weeks, the SGX945 group showed a 40% improvement relative to placebo, comparable to the 37% improvement seen with the approved drug apremilast, suggesting SGX945's competitive edge in the market.
- Patient Feedback: Patients reported reduced duration and number of oral ulcers during treatment, with SGX945 showing no treatment-related adverse events, indicating strong tolerability and potential for broader patient adoption.
- Future Development Plans: Soligenix intends to reformulate SGX945 for home-based treatment via subcutaneous injection, aiming to expand its application in innate immune-related inflammatory conditions, thereby enhancing its market competitiveness.
Trial Results: Soligenix, Inc. announced extended results from its Phase 2a trial of SGX302 (synthetic hypericin) for mild-to-moderate psoriasis, showing improvements in various clinical measures with a new gel formulation that enhances application ease.
Patient Tolerance: The SGX302 gel therapy was well tolerated by all patients, with no drug-related adverse events reported, and one patient achieved a significant improvement in their psoriasis status.
Comparative Outcomes: The results from the gel formulation were comparable to or improved upon those from the previous ointment formulation, indicating effective treatment potential for psoriasis lesions.
Stock Performance: Following the announcement, Soligenix's stock experienced a decline of 21.05%, trading at $1.21.








