Cytokinetics Faces Shareholder Investigation Over Alleged Misleading Statements
- Shareholder Investigation Initiated: Grabar Law Office is investigating whether Cytokinetics' executives breached their fiduciary duties, concerning shareholders who purchased shares before December 27, 2023, which could expose the company to legal and financial risks.
- FDA Review Delay: Cytokinetics disclosed on May 1, 2025, that the FDA postponed the review date for its New Drug Application from September 26, 2025, to December 26, 2025, indicating the company failed to provide necessary risk evaluation strategies in a timely manner, potentially affecting its market credibility.
- Impact of Misleading Statements: Company executives acknowledged that they submitted the NDA without including risk evaluation and mitigation strategies, resulting in significant shareholder losses, which may lead to broader legal liabilities and a decline in market confidence.
- Potential Legal Consequences: Shareholders can seek corporate reforms and fund recovery, indicating that the company's governance structure may face significant challenges, impacting future financing and operational capabilities.
Trade with 70% Backtested Accuracy
Analyst Views on CYTK
About CYTK
About the author

- EU Approval: Cytokinetics' MYQORZO (aficamten) has received approval from the European Commission for the treatment of adult symptomatic obstructive hypertrophic cardiomyopathy (oHCM), marking a significant milestone in the cardiovascular drug sector.
- Clinical Trial Results: Based on the positive outcomes from the SEQUOIA-HCM trial, the drug significantly improved patients' exercise capacity over 24 weeks, with pVO2 increasing by 1.76 mL/kg/min compared to 0.0 mL/kg/min in the placebo group, demonstrating robust efficacy.
- Market Launch Plans: MYQORZO is expected to launch in Germany in Q2 2026, available in 5mg, 10mg, 15mg, and 20mg formulations, aiming to provide flexible treatment options for patients with varying severity of symptoms.
- Patient Hope: The approval brings new treatment options for patients with hypertrophic cardiomyopathy, reflecting a commitment to improving patient quality of life and laying the groundwork for Cytokinetics' expansion in the global market.
- Grant Program Overview: Cytokinetics announced the recipients of its 2026 Communications Grant Program, aimed at supporting patient advocacy organizations for hypertrophic cardiomyopathy (HCM), reflecting the company's commitment to patient-centered communication.
- Camp Taylor Initiative: Camp Taylor will utilize the grant to launch 'Living with HCM: Youth Voices from Camp Taylor,' a digital communications project featuring a five-part video series and toolkit designed to raise awareness about HCM and assist healthcare providers in connecting with newly diagnosed families.
- SADS Foundation Campaign: The Canadian SADS Foundation will develop 'Think. Know. Act.: Cardiomyopathy Awareness in Canada,' a six-month digital campaign funded by the grant, aimed at increasing awareness of HCM symptoms, diagnosis, and management while amplifying patient voices and fostering community engagement.
- Grant Program History: The Cytokinetics Communications Grant Program has been running for eight years, providing resources to patient advocacy organizations to enhance their outreach and community engagement, thereby increasing disease awareness and supporting patients and their families.
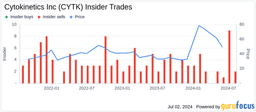
- FDA Review Extension: On May 1, 2025, Cytokinetics disclosed that the FDA extended the review period for its cardiac myosin inhibitor aficamten by three months due to additional time needed for the Risk Evaluation and Mitigation Strategy (REMS), causing a 12.9% drop in stock price to $37.35 per share on May 2, severely impacting investor confidence.
- NDA Submission Lacks REMS: The company admitted to multiple pre-NDA meetings with the FDA but chose to submit the NDA without an accompanying REMS, relying on labeling and voluntary education materials, which raised concerns about its safety monitoring capabilities and led to a further 2.7% decline in stock price to $33.04 per share on May 7.
- Investor Losses Intensify: The ongoing stock price decline has resulted in significant losses for Cytokinetics investors, particularly as the company failed to effectively manage communications and risk assessments with the FDA, potentially leading to future legal actions and a crisis of trust.
- Legal Investigation Initiated: The Law Offices of Frank R. Cruz are investigating whether Cytokinetics' board breached its fiduciary duties to shareholders, and if substantiated, this could have profound implications for the company's governance structure and future shareholder rights.

Stock Picks Overview: The second installment of the 2026 Barron’s Roundtable features 30 stock picks from five investment experts, highlighting both well-known companies and lesser-known options.
Familiar Names Included: Among the stock picks are recognizable brands such as Home Depot, Starbucks, and Nike, indicating a mix of established and emerging investment opportunities.
Biodegradable Innovation: The article hints at a British company that has recently acquired a manufacturer of biodegradable sneaker midsoles, showcasing a trend towards sustainable products in the footwear industry.
Investment Insights: The Roundtable serves as a platform for sharing diverse investment strategies and insights, appealing to both seasoned investors and those looking to explore new market trends.
- Cytokinetics Options Activity: Cytokinetics Inc (CYTK) has seen an options volume of 11,482 contracts today, representing approximately 1.1 million underlying shares, which is 51.5% of its average daily trading volume over the past month, indicating heightened market interest in its future performance.
- High Call Option Volume: Within CYTK, the $70 strike call option expiring on May 15, 2026, has traded 5,012 contracts, representing about 501,200 shares, suggesting increased investor expectations for the stock's upward movement.
- FuboTV Options Activity: FuboTV Inc (FUBO) has recorded an options volume of 51,955 contracts today, equating to approximately 5.2 million underlying shares, which constitutes 50.5% of its average daily trading volume over the past month, reflecting strong market interest in its stock.
- High Put Option Volume: For FUBO, the $2.50 strike put option expiring on January 16, 2026, has seen 28,711 contracts traded, representing around 2.9 million shares, indicating investor concerns about potential declines in the stock's future value.

- Shareholder Investigation Initiated: Grabar Law Office is investigating whether Cytokinetics' executives breached their fiduciary duties, concerning shareholders who purchased shares before December 27, 2023, which could expose the company to legal and financial risks.
- FDA Review Delay: Cytokinetics disclosed on May 1, 2025, that the FDA postponed the review date for its New Drug Application from September 26, 2025, to December 26, 2025, indicating the company failed to provide necessary risk evaluation strategies in a timely manner, potentially affecting its market credibility.
- Impact of Misleading Statements: Company executives acknowledged that they submitted the NDA without including risk evaluation and mitigation strategies, resulting in significant shareholder losses, which may lead to broader legal liabilities and a decline in market confidence.
- Potential Legal Consequences: Shareholders can seek corporate reforms and fund recovery, indicating that the company's governance structure may face significant challenges, impacting future financing and operational capabilities.






