Soligenix Announces Formation of European Medical Advisory Board for Cutaneous T-Cell Lymphoma
Phase 3 Clinical Study Announcement: Soligenix, Inc. is set to initiate a confirmatory Phase 3 clinical study of HyBryte™ (synthetic hypericin) for treating early-stage cutaneous T-cell lymphoma (CTCL) in late 2024, following positive feedback from the European Medicines Agency on its study design.
Medical Advisory Board Formation: The company has established a European Medical Advisory Board comprising leading CTCL experts to provide strategic guidance for the upcoming study, which aims to enroll approximately 80 patients across the U.S. and Europe, with results expected by mid-2026.
Get Free Real-Time Notifications for Any Stock
Analyst Views on SNGX
About SNGX
About the author

Soligenix Publishes Positive Phase 2a Results for SGX945 in Behçet's Disease Treatment
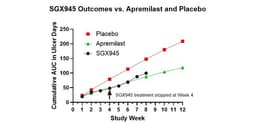
- Clinical Trial Results: Soligenix's SGX945 demonstrated significant improvement in 7 out of 8 patients in a Phase 2a trial for Behçet's Disease, indicating potential enduring effects after 4 weeks of treatment, which could enhance patient quality of life.
- Efficacy Comparison: After 4 weeks, the SGX945 group showed a 40% improvement relative to placebo, comparable to the 37% improvement seen with the approved drug apremilast, suggesting SGX945's competitive edge in the market.
- Patient Feedback: Patients reported reduced duration and number of oral ulcers during treatment, with SGX945 showing no treatment-related adverse events, indicating strong tolerability and potential for broader patient adoption.
- Future Development Plans: Soligenix intends to reformulate SGX945 for home-based treatment via subcutaneous injection, aiming to expand its application in innate immune-related inflammatory conditions, thereby enhancing its market competitiveness.
Soligenix Expands Psoriasis Trial, Revealing Improved Results with New Gel
Trial Results: Soligenix, Inc. announced extended results from its Phase 2a trial of SGX302 (synthetic hypericin) for mild-to-moderate psoriasis, showing improvements in various clinical measures with a new gel formulation that enhances application ease.
Patient Tolerance: The SGX302 gel therapy was well tolerated by all patients, with no drug-related adverse events reported, and one patient achieved a significant improvement in their psoriasis status.
Comparative Outcomes: The results from the gel formulation were comparable to or improved upon those from the previous ointment formulation, indicating effective treatment potential for psoriasis lesions.
Stock Performance: Following the announcement, Soligenix's stock experienced a decline of 21.05%, trading at $1.21.









